Reflected shock tube studies of high-temperature rate constants for OH + C2H2 and OH + C2H4†
Abstract
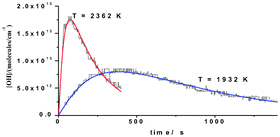
The reflected shock tube technique with multi-pass absorption spectrometric detection of OH-radicals at 308 nm (corresponding to a total path length of ∼4.9 m) has been used to study the reactions, OH + C2H2
→ products (1) and OH + C2H4
→ C2H3 + H2O (2). The present optical configuration gives a S/N ratio of ∼1 at ∼0.5−1.0 × 1012 radicals cm−3. Hence, kinetics experiments could be performed at [OH]0 = ∼4–20 ppm thereby minimizing secondary reactions. OH was produced rapidly from the dissociations of either CH3OH or NH2OH (

 Please wait while we load your content...
Please wait while we load your content...