Kinetics and mechanism of the reactions of CH3CO and CH3C(O)CH2 radicals with O2. Low-pressure discharge flow experiments and quantum chemical computations†
Abstract
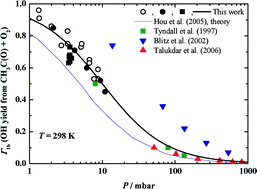
The reactions CH3CO + O2 → products (1), CH3CO + O2 → OH +other products (1b) and CH3C(O)CH2 + O2 → products (2) have been studied in isothermal discharge flow reactors with laser induced fluorescence monitoring of OH and CH3C(O)CH2 radicals. The experiments have been performed at overall pressures between 1.33 and 10.91 mbar of helium and 298 ± 1 K reaction temperature. OH formation has been found to be the dominant reaction channel for CH3CO + O2: the branching ratio, Γ1b = k1b/k1, is close to unity at around 1 mbar, but decreases rapidly with increasing pressure. The rate constant of the overall reaction, k2, has been found to be pressure dependent: the fall-off behaviour has been analysed in comparison with reported data. Electronic structure calculations have confirmed that at room temperature the reaction of CH3C(O)CH2 with O2 is essentially a recombination-type process. At high temperatures, the further reactions of the acetonyl–peroxyl adduct may yield OH radicals, but the most probable channel seems to be the O2-catalysed keto–enol transformation of acetonyl. Implications of the results for atmospheric modelling studies have been discussed.

 Please wait while we load your content...
Please wait while we load your content...