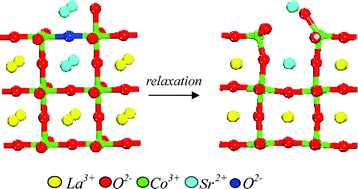
Atomistic computational modelling of the surface structure of the catalytically-active perovskite LaCoO3 has been undertaken in order to develop better models of the processes involved during catalytic oxidation processes. In particular, the energetics of creating oxygen ion vacancies at the surface have been investigated for the three low index faces (100), (110) and (111). Two mechanisms for vacancy creation have been considered involving dopant Sr2+ cations at the La3+ site and reduction of Co3+ to Co2+. For both mechanisms, there is a general tendency that the smaller the cation defect separation, the lower the energy of the cluster, as would be expected from simple electrostatic considerations. In addition, there are clear indications that oxygen vacancies are more easily created at the surface than in the bulk. The results also confirm that the presence of defects strongly influences crystal morphology and surface chemistry. The importance of individual crystal surfaces in catalysis is discussed in terms of the energetics for the creation of oxygen vacancies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...