An NMR strategy for obtaining multiple conformational constraints for 15N–13C spin-pair labelled organic solids
Abstract
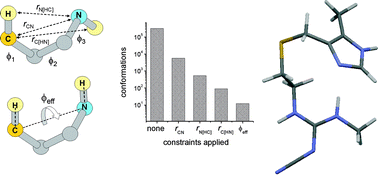
This work demonstrates a solid-state NMR strategy for extracting multiple interatomic distance and angle constraints from moderately complex solid organic compounds containing a single 13C label and a single 15N label. It is shown that the constraints obtained for the compound cimetidine, with the

 Please wait while we load your content...
Please wait while we load your content...