O- vs. N-protonation of 1-dimethylaminonaphthalene-8-ketones: formation of a peri N–C bond or a hydrogen bond to the pi-electron density of a carbonyl group†
Abstract
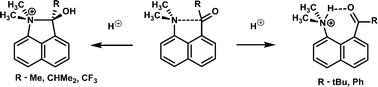
X-ray crystallography and solid-state NMR measurements show that protonation of a series of 1-dimethylaminonaphthalene-8-ketones leads either to O protonation with formation of a long N–C bond (1.637–1.669 Å) between peri groups, or to N protonation and formation of a hydrogen bond to the π surface of the carbonyl group, the latter occurring for the larger ketone groups (C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)R, R = t-butyl and phenyl). Solid state 15N MAS NMR studies clearly differentiate the two series, with the former yielding significantly more deshielded resonances. This is accurately corroborated by DFT calculation of the relevant chemical shift parameters. In the parent ketones X-ray crystallography shows that the nitrogen lone pair is directed towards the carbonyl group in all cases.
O)R, R = t-butyl and phenyl). Solid state 15N MAS NMR studies clearly differentiate the two series, with the former yielding significantly more deshielded resonances. This is accurately corroborated by DFT calculation of the relevant chemical shift parameters. In the parent ketones X-ray crystallography shows that the nitrogen lone pair is directed towards the carbonyl group in all cases.


 Please wait while we load your content...
Please wait while we load your content...