Secondary nucleation-mediated effects of stirrer speed and growth rate on induction time for unseeded solution
Abstract
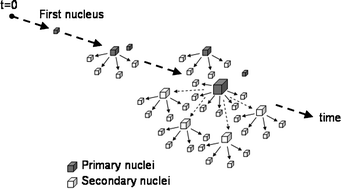
Simulation of induction time for nucleation was performed under isothermal conditions for unseeded aqueous solutions of different concentrations. The effects of stirrer speed and growth rate on induction time were both explained with the proposed secondary nucleation-mediated mechanism. The effect of stirrer speed Nr was incorporated into the simulation with an empirical relation of kb2 ∝ Nrj, where kb2 is the coefficient in the secondary nucleation rate equation B2 = kb2 (ΔT)b2μ3, ΔT is supercooling, j and b2 are empirical constants and μ3 is the third moment of crystal size distribution. The simulated induction time decreased with an increase in stirrer speed at lower supercoolings, while it remained unchanged at higher supercoolings. Such action of stirrer speed was similar to that observed in the literature data. The growth rate effect was considered to be caused by a faster increase in the secondary nucleation rate via a faster increase in μ3. The simulated induction time decreased with an increase in crystal growth rate. This type of growth rate effect is completely different from the existing mechanism considering the time needed for invisible nuclei to grow to a detectable size.

 Please wait while we load your content...
Please wait while we load your content...