Surprisingly complex supramolecular behaviour in the crystal structures of a family of mono-substituted salicylic acids†
Abstract
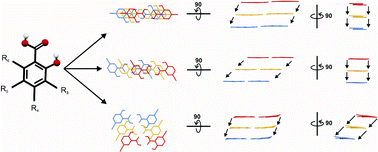
Crystal structure packing analyses of a family of monosubstituted salicylic acids, including some ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O(H)⋯H–O(carbox) single bridge. In the 4-ACM structure, the
O⋯H–O(H)⋯H–O(carbox) single bridge. In the 4-ACM structure, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O(carbox) and (ACM–Me)C–H⋯O
O⋯H–O(carbox) and (ACM–Me)C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(carbox) double bridges, whilst the 6-MeO molecules, in which the carboxyl OH group is H-bonding intramolecularly to the MeO oxygen, forming catemers via bifurcating carboxyl OH and C
C(carbox) double bridges, whilst the 6-MeO molecules, in which the carboxyl OH group is H-bonding intramolecularly to the MeO oxygen, forming catemers via bifurcating carboxyl OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functions. The remaining outlier is the 5-amino (5-NH2) derivative, which exists in a zwitterionic form, with one carboxyl oxygen forming a short O⋯H–N intermolecular bond to an –NH3+ group, whilst the second oxygen forms three longer O⋯H–N bonds to three different –NH3+ groups. The larger, “normal” group of 17 derivatives adopt 16 different structures, generally comprising stacks of molecules with varying relative shifts of the molecular planes, and interactions involving the substituents. Surprisingly, the family contains only two polymorphic pairs, 5-Br α, β and 5-I α, β, in each of which the forms show low similarities, but two isostructures, the 5-Br α and 5-I β forms.
O functions. The remaining outlier is the 5-amino (5-NH2) derivative, which exists in a zwitterionic form, with one carboxyl oxygen forming a short O⋯H–N intermolecular bond to an –NH3+ group, whilst the second oxygen forms three longer O⋯H–N bonds to three different –NH3+ groups. The larger, “normal” group of 17 derivatives adopt 16 different structures, generally comprising stacks of molecules with varying relative shifts of the molecular planes, and interactions involving the substituents. Surprisingly, the family contains only two polymorphic pairs, 5-Br α, β and 5-I α, β, in each of which the forms show low similarities, but two isostructures, the 5-Br α and 5-I β forms.

 Please wait while we load your content...
Please wait while we load your content...