Polymorphism or pseudopolymorphism of a macrocyclic compound: helical structure, layered structure and pseudorotaxane constructed by weak intermolecular interactions†
Abstract
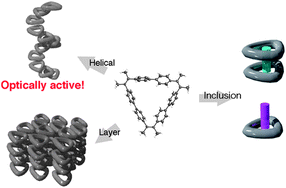
Molecules of macrocyclic aromatic hydrocarbon 1 assembled to form various three-dimensional (3D) structures via weak intermolecular interactions, including aromatic–aromatic interactions (1a–d). In the crystal obtained from a

 Please wait while we load your content...
Please wait while we load your content...