Palladium-catalyzed enol/enolate directed oxidative annulation: functionalized naphthofuroquinone synthesis and bioactivity evaluation†
Abstract
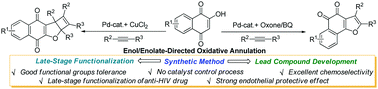
A palladium-promoted oxidative annulation reaction for the synthesis of structurally diverse naphthoquinone-containing heterocycles has been developed, providing switchable access to 1,2-naphthofuroquinones and densely functionalized cyclobutene-fused 1,4-naphthofuroquinones by selective enol/enolate-directed processes. The synthetic application was extended by late-stage functionalization of an anti-HIV drug. The practical value of 1,2-naphthofuroquinone synthesis was highlighted in endothelial protective lead compound development.


 Please wait while we load your content...
Please wait while we load your content...