Alkyne aza-Prins cyclization of N-(hexa-3,5-diynyl)tosylamides with aldehydes using triflic acid and a binuclear aluminum complex†
Abstract
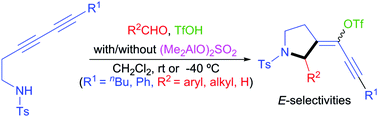
The alkyne aza-Prins cyclization of 3,5-diynyl amides and various aldehydes was developed using TfOH with/without (Me2AlO)2SO2. This method, which could be applied to homopropargyl amides, provides TfO-substituted pyrrolidines with E-selectivities and exclusive regioselectivities. This work represents a first example of the aza-Prins cyclization with the introduction of TfO groups.


 Please wait while we load your content...
Please wait while we load your content...