A general diversity oriented synthesis of asymmetric double-decker shaped silsesquioxanes†
Abstract
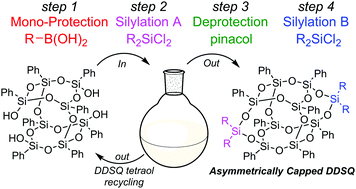
A strategically novel synthesis of nano-sized, asymmetrically functionalized double-decker shaped silsesquioxanes (DDSQ) is reported. Selective protection with a boronic acid affords the crucial mono-protected intermediate en route to the asymmetric products. Generation of symmetric by-products is minimized by judicious choice of base, and high recovery of recyclable starting DDSQ tetraol is achieved.


 Please wait while we load your content...
Please wait while we load your content...