Cu-catalyzed regioselective borylcyanation of 1,3-dienes†
Abstract
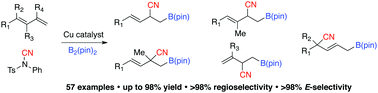
Catalytic regioselective generation of an allyl–Cu complex through Cu–B(pin) (pin = pinacolato) addition to 1,3-dienes followed by reaction with an electrophilic cyanation reagent to afford multifunctional organoboron compounds is presented. Reactions of a wide range of 1,3-dienes with different substitution patterns promoted by an easily accessible phosphine–Cu complex proceed with high yields and regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...