Intramolecular cycloaddition/rearrangement cascade from gold(iii)-catalysed reactions of propargyl aryldiazoesters with cinnamyl imines†
Abstract
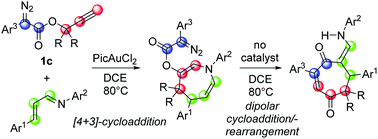
Conjugated cycloheptene-1,4-dione-enamines are cycloaddition products from a surprising rearrangement in a Au(III)-catalysed reaction between propargyl aryldiazoacetates and cinnamyl imines. This complex transformation occurs through an initial rapid Au(III)-catalysed [4+3]-cycloaddition to form dihydroazepinyl aryldiazoacetates followed by a subsequent uncatalyzed domino transformation that occurs by sequential [3+2]-cycloaddition-/nitrogen extrusion and acyloxy migration/retro-Michael addition/tautomerization.


 Please wait while we load your content...
Please wait while we load your content...