Experimental and computational studies on H2O-promoted, Rh-catalyzed transient-ligand-free ortho-C(sp2)–H amidation of benzaldehydes with dioxazolones†
Abstract
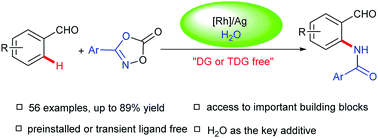
An efficient and convenient ligand-free, rhodium-catalyzed ortho-C(sp2)–H amidation of benzaldehydes with dioxazolones using H2O as the key promoter is described. Using this protocol, a wide range of benzaldehyde substrates were selectively amidated in good to excellent yields with broad functional group compatibility. KIE experiments revealed that the C–H bond activation was likely the rate-limiting step. In addition, computational studies indicated that the catalyst precursor interacted with water and dioxazolones to generate the active catalytic species. Notably, the practicality and efficacy of this method were illustrated by a late-stage amidation of an estrone-derived molecule and further transformations of the amidated product.


 Please wait while we load your content...
Please wait while we load your content...