Diversion of a thioglycoligase for the synthesis of 1-O-acyl arabinofuranoses†
Abstract
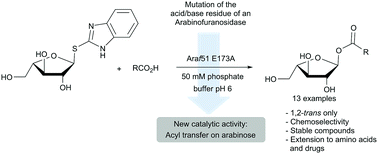
An arabinofuranosylhydrolase from the GH51 family was transformed into an acyl transferase by mutation of the catalytic acid/base amino acid. The resulting enzyme was able to transfer carboxylic acid onto the anomeric position of arabinose with complete chemo- and stereoselectivity. A wide range of acyl α-L-arabinofuranoses was obtained with yields ranging from 25 to 83%. Using this method, ibuprofen and N-Boc phenylalanine were successfully transformed into their corresponding acyl conjugates, expanding the scope of the reaction to drugs and amino acids.


 Please wait while we load your content...
Please wait while we load your content...