Synthetic studies on daphniglaucins†
Abstract
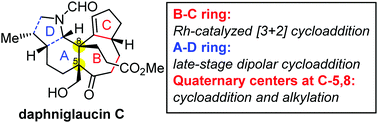
The synthetic approach to the core framework of daphniglaucin-type Daphniphyllum alkaloids is described herein. The B–C ring was constructed via a Rh-catalyzed [3+2] cycloaddition. Continuous quaternary centers were installed through [3+2] cycloaddition and alkylation. The attempt to build the A–D ring motif using dipolar cycloaddition of azomethine ylides was extensively investigated.


 Please wait while we load your content...
Please wait while we load your content...