Mass spectrometry captures structural intermediates in protein fiber self-assembly†
Abstract
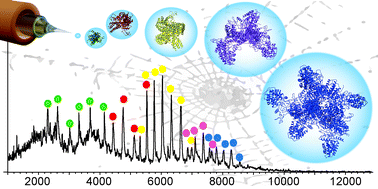
Self-assembling proteins, the basis for a broad range of biological scaffolds, are challenging to study using most structural biology approaches. Here we show that mass spectrometry (MS) in combination with MD simulations captures structural features of short-lived oligomeric intermediates in spider silk formation, providing direct insights into its complex assembly process.


 Please wait while we load your content...
Please wait while we load your content...