Rh(iii)-catalyzed C–H activation reactions forming 1H-isoindoles containing a quaternary carbon center from aryl ketones or benzylamines†
Abstract
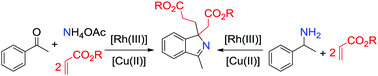
Rh(III)/Cu(OAc)2 catalyzed, one-pot reactions of aryl ketones, acrylate esters and ammonium acetate or α-substituted benzylamines under microwave irradiation conditions produce 1H-isoindoles bearing a quarternary carbon center.

 Please wait while we load your content...
Please wait while we load your content...