Photoswitchable ring-opening polymerization of lactide catalyzed by azobenzene-based thiourea†
Abstract
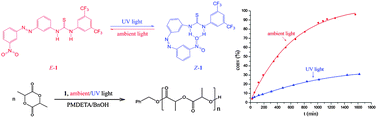
The reactivity of a catalytic polymerization system using photoresponsive azobenzene-based thiourea/PMDETA as a catalyst could be switched between slow and fast states by alternating exposure to UV and ambient light, because the active site of azobenzene thiourea is blocked via intramolecular hydrogen bonding when the azobenzene thiourea transfers from the E isomer to the Z isomer under UV irradiation.

 Please wait while we load your content...
Please wait while we load your content...