Self-assembled ion-pair organocatalysis – asymmetric Baeyer–Villiger oxidation mediated by flavinium–cinchona alkaloid dimer†
Abstract
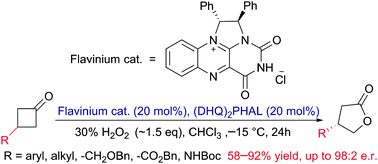
An ion-pair catalyst generated by assembly of a chiral flavinium and a cinchona alkaloid dimer for use in asymmetric Baeyer–Villiger oxidation is presented. Ion-pair formation is essential for enhancing the catalytic activity and stereoselectivity. The catalyst is applicable to structurally diverse 3-substituted cyclobutanones, providing good to excellent enantioselectivities (up to 98 : 2 e.r.). This study provides the first example of self-assembly of a flavin derivative and a base to form a chiral reaction site that enables a highly stereoselective reaction to occur.

 Please wait while we load your content...
Please wait while we load your content...