Copper-catalyzed cross-coupling reactions of epoxides with gem-diborylmethane: access to γ-hydroxyl boronic esters†
Abstract
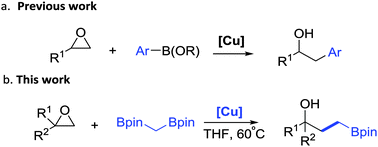
Herein, we describe a novel copper-catalyzed epoxide opening reaction with gem-diborylmethane. Aliphatic, aromatic epoxides as well as aziridines are converted to the corresponding γ-pinacolboronate alcohols or amines in moderate to excellent yields. This new reaction provides beneficial applications for classic epoxide substrates as well as interesting gem-diborylalkane reagents.

 Please wait while we load your content...
Please wait while we load your content...