Electroorganic synthesis of nitriles via a halogen-free domino oxidation–reduction sequence†
Abstract
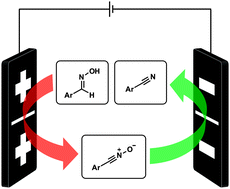
A direct electroorganic sequence yielding nitriles from oximes in undivided cells is reported. Despite the fact that intermediate nitrile oxides might be formed, the method is viable to prepare benzonitriles without substituents ortho to the aldoxime moiety. This constant current method is easy to perform for a broad scope of substrates and employs common electrodes, such as graphite and lead.

 Please wait while we load your content...
Please wait while we load your content...