Synthesis of a diboryl-N-heterocycle and its conversion to a bidentate cationic Lewis acid†
Abstract
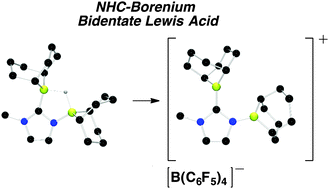
Sequential reaction of 2-lithio-1-methylimidazole with 9-borabicyclo[3.3.1]nonane (9-BBN) dimer and 9-Cl-9-BBN yields diboryl-N-heterocycle C4H5N2(H)(BC8H14)2 (1). Reaction of 1 with I2 results in the net substitution of chelated hydride for a singly boron-bound iodide to produce C4H5N2(I)(BC8H14)2 (2). Conversely, reaction of 1 with [Ph3C][B(C6F5)4] results in the formation of the bidentate cationic Lewis acid [(C4H5N2)(BC8H14)2][B(C6F5)4] (3). Compound 3 catalyzes the hydrogenation of N-benzylidene-tert-butylamine at room-temperature.

 Please wait while we load your content...
Please wait while we load your content...