The Mukaiyama aldol reaction of in situ generated nitrosocarbonyl compounds: selective C–N bond formation and N–O bond cleavage in one-pot for α-amination of ketones†
Abstract
A practical protocol for the α-amination of ketones (up to 99% yield) has been developed via the Mukaiyama aldol reaction of in situ generated nitrosocarbonyl compounds. The reaction with silyl enol ethers having a disilane (−SiMe2TMS) backbone proceeded not only with perfect N-selectivity but concomitant N–O bond cleavage was also accomplished. Such a cascade of C–N bond formation and N–O bond cleavage in a single step was heretofore unknown in the field of nitrosocarbonyl chemistry. A very high diastereoselectivity (dr = 19 : 1) was accomplished using (−)-menthol derived chiral nitrosocarbonyl compounds.
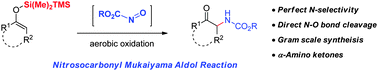

 Please wait while we load your content...
Please wait while we load your content...