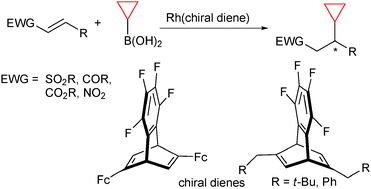
Enantioselective 1,4-addition of cyclopropylboronic acid catalyzed by rhodium/chiral diene complexes†
Abstract
Rhodium-catalyzed asymmetric addition of cyclopropylboronic acids to electron-deficient alkenes such as alkenylsulfones, enones, enoates, and nitroalkenes proceeded to give high yields of the corresponding 1,4-addition products with high enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...