Abstract
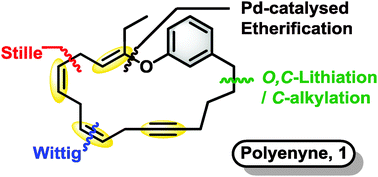
The stereoselective synthesis of a challenging macrocyclic polyene scaffold, containing a sensitive vinyl ether motif, has been accomplished using O,C-dilithiation/selective C-alkylation, Pd-catalysed etherification and Wittig reactions as key steps. An end-game macrocyclisation strategy employed a regio- and stereoselective Stille cross-coupling using Pd(Br)(N-Succ)(AsPh3)2 (AsCat) as the precatalyst.

 Please wait while we load your content...
Please wait while we load your content...