Revisiting the assembly of amino ester-based benzene-1,3,5-tricarboxamides: chiral rods in solution†
Abstract
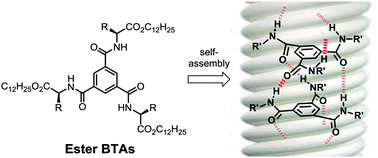
Some benzene-1,3,5-tricarboxamide (BTA) monomers derived from (L) α-amino esters self-assemble into long rods at millimolar concentrations, and display a strong chiral amplification effect. These rods are in competition with dimeric species.

 Please wait while we load your content...
Please wait while we load your content...