β,γ-Bis-substituted PNA with configurational and conformational switch: preferred binding to cDNA/RNA and cell-uptake studies†
Abstract
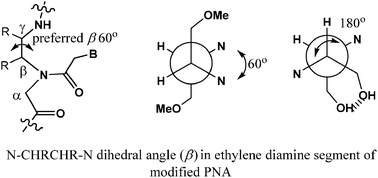
(S,S)- and (R,R)-β,γ-Bis-substituted PNAs were synthesized from the C-2 symmetric vicinal diamine system embedded in 1,4 dihydroxybutane and 1,4-dimethoxybutane scaffolds. (R,R)-β,γ-Bis-methoxymethyl-PNA derived from D-tartaric acid was found to be in the right configuration and conformation to be an excellent mimic of PNA, endowed with superior ability to enter into cells.

 Please wait while we load your content...
Please wait while we load your content...