Sequential one-pot multienzyme (OPME) synthesis of lacto-N-neotetraose and its sialyl and fucosyl derivatives†
Abstract
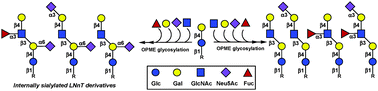
Lacto-N-neotetraose and its sialyl and fucosyl derivatives including Lewis x (Lex) pentasaccharide, sialyl Lewis x (sLex) hexasaccharide and internally sialylated derivatives were enzymatically synthesized from readily available lactoside, commercially available uridine 5′-diphosphate-glucose (UDP-Glc) and the corresponding monosaccharides using a highly efficient sequential one-pot multienzyme (OPME) strategy. The OPME strategy which combines bacterial glycosyltransferases and sugar nucleotide generation enzymes provides easy access to the biologically important complex oligosaccharides at preparative scale. Moreover, the same OPME strategy can be used for the regioselective introduction of sialic acid to the internal galactose unit of LNnT in a designed glycosylation route by simply changing the glycosylation sequence.

 Please wait while we load your content...
Please wait while we load your content...