Borylstannylation of alkynes with inverse regioselectivity: copper-catalyzed three-component coupling using a masked diboron†
Abstract
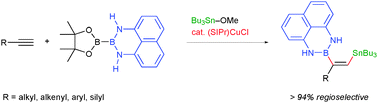
A variety of terminal alkynes are facilely convertible into cis-boryl(stannyl)alkenes with inverse regioselectivity to those of the previous borylstannylation by the copper-catalyzed three-component reaction using a masked diboron. The synthetic utility of the resulting boryl(stannyl)alkenes has been demonstrated by chemoselective coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...