Carbon-centered radicals add reversibly to histidine – implications†
Abstract
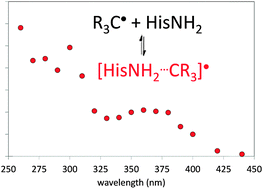
Carbon-centered radicals of alcohols commonly used as hydroxyl radical scavengers (MeOH, EtOH, i-PrOH and t-BuOH) add reversibly to histidine with equilibrium constants up to 3 × 103 M−1 and rate constants on the order of 109 M−1 s−1. Similar equilibria may compromise determinations of one-electron (radical) electrode potentials.

 Please wait while we load your content...
Please wait while we load your content...