An unprecedented Pd-catalyzed trans-addition of boronic acids to ynamides†
Abstract
An unprecedented Pd-catalyzed trans-addition of boronic acids to ynamides has been reported, giving α,β-disubstituted enamides in high yields with excellent regio- and stereoselectivity. A possible mechanism involving the palladium carbene intermediate has been proposed to account for the unusual trans-addition.
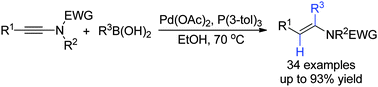

 Please wait while we load your content...
Please wait while we load your content...