Direct access to isoindolines through tandem Rh(iii)-catalyzed alkenylation and cyclization of N-benzyltriflamides†
Abstract
The rhodium-catalyzed oxidative alkenylation of N-benzyltriflamides with olefins followed by an intramolecular cyclization via C–H bond activation is described. This method results in the direct and efficient synthesis of highly substituted isoindoline frameworks.
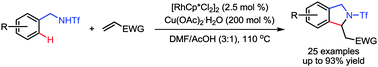

 Please wait while we load your content...
Please wait while we load your content...