A structural model for glutathione-complexed iron–sulfur cluster as a substrate for ABCB7-type transporters†
Abstract
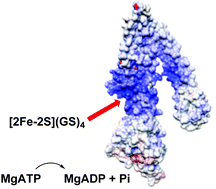
Glutathione-complexed [2Fe–2S] cluster is shown to significantly stimulate the ATPase activity of an ABCB7-type transporter in both solution and proteoliposome-bound forms (KD ∼ 68 μM). The cluster is a likely natural substrate for this transporter, which has been implicated in cytosolic Fe–S cluster protein maturation. A possible substrate-binding site is identified on a new structural model for the active transporter.

 Please wait while we load your content...
Please wait while we load your content...