Silver-catalyzed dynamic systemic resolution of α-iminonitriles in a 1,3-dipolar cycloaddition process†
Abstract
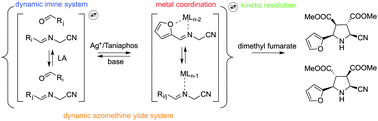
A dynamic azomethine ylide system was established using Sc(OTf)3 and Ag/Taniaphos as catalysts. The system was subsequently kinetically resolved in a tandem 1,3-dipolar cycloaddition process where the silver complex acted as both a reaction catalyst and an external selector, resulting in the formation of an exclusive pyrrolidine product in good yield and enantiopurity.
- This article is part of the themed collection: Systems Chemistry

 Please wait while we load your content...
Please wait while we load your content...