A novel asymmetric route to succinimides and derived compounds: synthesis of the lignan lactone (+)-hinokinin
Abstract
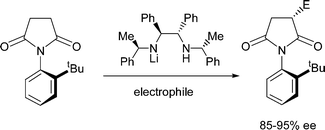
A novel approach to chiral succinimides and derived compounds has been developed that involves chiral lithium amide desymmetrisation of an N-ortho-tert-butylphenyl succinimide to generate a putative atropisomeric intermediate enolate,

 Please wait while we load your content...
Please wait while we load your content...