A novel quantitative 31P NMR spectroscopic analysis of hydroxyl groups in lignosulfonic acids†
Abstract
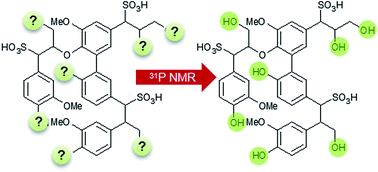
31P nuclear magnetic resonance (NMR) spectroscopy is a common analytical approach for the quantitative determination of the different hydroxyl groups in technical lignins. However, the analysis of lignosulfonates with this method is more difficult due to solubility problems in the commonly used solvent systems. Hence, extensive derivatization procedures must be applied to facilitate a quantitative hydroxyl group analysis of the sulfonated lignins. In this study, we present an alternative and facile approach for the quantitative analysis of the hydroxylic environment in lignosulfonates. Technical lignosulfonates were transferred into the corresponding lignosulfonic acids by ion exchange treatment. After lyophilization, the samples were dissolved in a novel solvent system consisting of DMF/DMF-d7/pyridine (4.5 : 1 : 1; v/v) in the presence of an internal standard (endo-N-hydroxy-5-norbornene-2,3-dicarboxylic acid imide), relaxation agent and derivatization reagent (2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane). The accuracy of the experimental protocol was shown by analysis of lignin model compounds and by comparison with previously published derivatization procedures.

 Please wait while we load your content...
Please wait while we load your content...