A weighting approach for chromatographic fingerprinting to ensure the quality consistency of botanical drug products†
Abstract
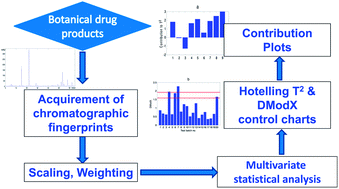
The batch-to-batch quality consistency needs to be controlled to ensure the efficacy and safety of botanical drug products. Chromatographic fingerprinting is a powerful tool for characterizing complex chemical systems such as botanical drugs. This paper presented the combined use of a proposed weighting algorithm and multivariate statistical analysis on fingerprint data to achieve quality consistency evaluation of botanical drug products. A set of fingerprint data following multivariate normal distribution was simulated based on the real fingerprint data of a botanical drug product, Danshen injection. The weight of each peak was set based on its batch-to-batch peak variation. A principal component analysis (PCA) model was established, and the Hotelling T2 and DModX statistics were applied to evaluate quality consistency. The effect of weighting on the monitoring capability of the two statistics was evaluated through monitoring simulated peak area variations. The results showed that both the two statistics were more sensitive to the characteristic peaks with smaller batch-to-batch peak variations. Hence the peaks with larger variability will be permitted to have a wider tolerance range of variation, while a more narrow range of variation was seen for the peaks with smaller variability among batches. Peak weighting combined with multivariate statistical analysis has overcome some drawbacks of the fingerprint similarity analysis method, and is worthy of application and recommendation for the quality consistency evaluation of botanical drug products.

 Please wait while we load your content...
Please wait while we load your content...