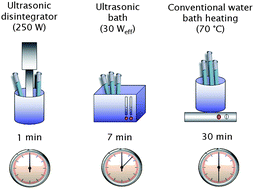
The aim of the presented study was to investigate the use of ultrasonic irradiation for the improvement of analytical methods. A procedure for boron determining based on the conversion of boric acid to tetrafluoroborate and subsequent spectrophotometric detection, previously reported from our laboratory, was used as a model. The optimum conditions for the conversion of boron to tetrafluoroborate anion (0.9 mol L−1 H2SO4, 0.1 mol L−1 F−, 7 min ultrasonication) as well as for complex formation and extraction of BF4− with DIC (1 × 10−4 mol L−1 DIC) were found. The absorbance of the coloured extracts obeys Beer's law in the range 0.1–1.0 mg L−1 of B. The limit of detection calculated from a blank test (n = 10; P = 0.95) based on 3 s is 0.02 mg L−1 of B. The procedure was applied to the analysis of mineral waters and a pharmaceutical preparative. The main advantage of ultasonification is that the conversion process occurs much faster; reaction time therefore decreases from 30 min to 7 min. Moreover, it does not lead to the heating of the sample, meaning, as a consequence, the value of the blank test decreases, and the reaction does not have to be performed in a closed system.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...