Recent progress in the synthesis of N-substituted arylamines by reductive cross-coupling of nitroarenes
Abstract
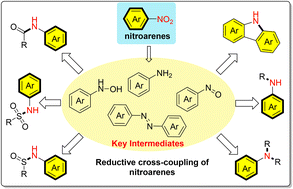
Highly functionalized N-substituted arylamines are the core structural motifs of numerous biologically active molecules, agrochemicals, and functional materials. Classically, using arylamines as nitrogen sources via transition metal-catalyzed cross-coupling reactions and substitution reactions of electrophilic reagents is the most common method used to obtain functionalized N-substituted arylamines. The direct reductive cross-coupling of nitroarenes is a more efficient protocol for the construction of N-substituted arylamine derivatives, as it avoids the protection and deprotection processes of arylamines, thereby achieving step economy. In addition, the use of distinct nitrogen-active intermediates, which are derived from the reduction of nitroarenes by using different reducing agents, makes it possible to precisely modulate both reactivity and selectivity. Recent direct reductive coupling processes of nitroarenes have considerably promoted the synthesis of highly functionalized N-substituted arylamine derivatives, including N-alkylanilines, diarylamines, and amides. The typical courses of two-component reductive couplings of nitroarenes include C(sp2)–NAr and C(sp3)–NAr couplings, and intermolecular reductive couplings of nitroarenes are further classified according to the coupling of nitroaromatics with carbonyl compounds, alkenes, arylboronic acids, aryl or alkyl halides, alcohols, and other substrates. In this review, we aim to present the current progress in research on the two-component intermolecular reductive cross-coupling of nitroarenes.
- This article is part of the themed collections: 2024 Organic Chemistry Frontiers Review-type Articles and 2024 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...