Nickel-catalyzed acylation of vinylpyridine with alkylzincs under 1 atm CO†
Abstract
A nickel-catalyzed acylation of vinylpyridines with CO at atmospheric pressure is reported, allowing for an expedient approach to synthesize β-acyl pyridine derivatives with high regio- and chemoselectivity. The electron-withdrawing property of pyridine plays pivotal roles in activating the alkenyl group, thereby facilitating this carbonylative process. In addition to vinylpyridines, other alkenylheterocycles such as thiazole and quinoline were also suitable for this method.
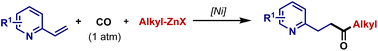


 Please wait while we load your content...
Please wait while we load your content...