Synthesis of 4-sulfonyl-1,7-diesters via K2CO3-mediated alkylative debenzoylation of α-sulfonyl o-hydroxyacetophenones with acrylates†
Abstract
The synthesis of 4-sulfonyl-1,7-diesters was well developed, under open-vessel conditions, by K2CO3-mediated double alkylation of α-sulfonyl o-hydroxyacetophenones with acrylates and tandem debenzoylation of the resulting α,α-disubstituted o-hydroxyacetophenones. A plausible mechanism is proposed and discussed here. This high-yielding protocol provides a highly effective intermolecular alkylation and intramolecular debenzoylation via the formation of two carbon–carbon (C–C) single bonds and the cleavage of a carbon–carbon (C–C) single bond.
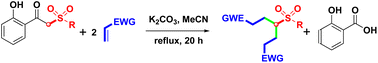


 Please wait while we load your content...
Please wait while we load your content...