Glycosylation of n-pentenyl glycosides using bromodiethylsulfonium salt as an activator: interception of the glycosyl intermediate by chloride ion transfer†
Abstract
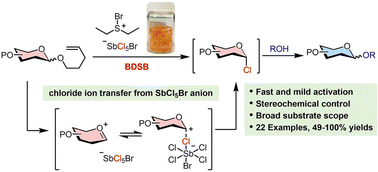
Utilization of n-pentenyl glycosides (NPGs) in modern carbohydrate synthesis may be hindered by their sluggish activation, which results from reversible halogenation and cyclization processes. Bromodiethylsulfonium bromopentachloroantimonate (BDSB) has been previously shown to be a powerful brominating agent for the cation–π polyene cyclization of less reactive and electron-poor polyenes. This study demonstrates the activation of NPGs using BDSB as a powerful brominating agent. BDSB effectively activates the terminal olefins of NPGs and the reaction proceeds through 5-exo-tet cyclization, offering a rapid and mild approach for glycosylation with a wide range of glycosyl donors, including n-pentenyl mannoside, n-pentenyl galactoside, and n-pentenyl glucoside. The success of this approach derives from the chloride ion transfer from the nonnucleophilic SbCl5Br anion to the glycosyl intermediate, which disrupts the equilibrium and produces a glycosyl chloride intermediate that is smoothly converted to 22 coupling products, with yields ranging from moderate to excellent (49–100%). The β-selective glycosylation is accomplished when employing NPGs equipped with a neighboring participating group. The practicality of the BDSB-activated glycosylation is demonstrated by a gram-scale synthesis. This study showcases BDSB as a potent activator for NPG glycosylation through the interception of a glycosyl intermediate that diminishes the equilibration during halogenation and 5-exo-tet cyclization.


 Please wait while we load your content...
Please wait while we load your content...