Theoretical investigation of tautomerism of 2- and 4-pyridones: origin, substituent and solvent effects†
Abstract
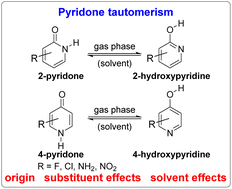
Computational investigation at the BHandHLYP/6-311+G(d,p) level of theory of the gas-phase tautomerism of 2- and 4-pyridones confirmed the slight prevalence of lactim in the case of the former, but its dominance in the case of the latter, as shown previously. Examination of aromaticity by using HOMA, EDDB, NBOdel, NICS and AICD led to the conclusion that tautomerization of 4-pyridone results in greater aromaticity gain. It is also driven by the Pauli repulsion relief, which was revealed by the tautomerization energy decomposition analysis. By contrast, in the case of 2-pyridone, lactim is favoured by orbital and electrostatic interactions and disfavoured by the Pauli repulsion. Aromaticity gain in this case is smaller. The position of the tautomeric equilibrium can be modulated by substituent inductive effects (Cl and F), inductive and resonance effects (NH2 and NO2), hydrogen bonding (NO2), and medium polarity, the increase of which increases lactam population.
- This article is part of the themed collection: Computational Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...