A convenient synthetic approach to highly hindered 3,3′-bis(2,4,6-tri-tert-butylphenyl)-BINOL-derived phosphoric acids†
Abstract
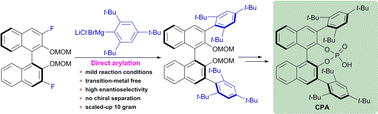
Considering the success of BINOL phosphoric acids and a serious lack of preparation methods for hindered chiral phosphoric acid catalysts, herein we present an efficient approach to highly hindered (S) and (R)-3,3′-bis(2,4,6-tri-tert-butylphenyl)-BINOL-derived phosphoric acids with high enantioselectivity. Direct arylations of the C–F bonds in optically pure 3,3′-difluoro-BINOL derivatives with (2,4,6-tri-tert-butylphenyl)magnesium bromide were explored as the key transformation. Optical resolution chromatography was avoided and both enantiomers were successfully prepared on a ten-gram-scale via our method.


 Please wait while we load your content...
Please wait while we load your content...