Enantioselective aromatic Claisen rearrangement of allyl 2-naphthyl ethers catalyzed by π–Cu(ii) complexes†
Abstract
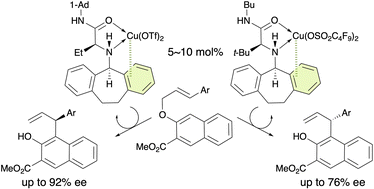
The first catalytic enantioselective aromatic Claisen rearrangement of allyl 2-naphthyl ethers using 5–10 mol% of π–copper(II) complexes is reported. A Cu(OTf)2 complex with an L-α-homoalanine amide ligand gave (S)-products in up to 92% ee. Conversely, a Cu(OSO2C4F9)2 complex with an L-tert-leucine amide ligand gave (R)-products in up to 76% ee. Density-functional-theory (DFT) calculations suggest that these Claisen rearrangements proceed stepwise via tight-ion-pair intermediates, and that (S)- and (R)-products are enantioselectively obtained via the staggered transition states for the cleavage of the C–O bond, which is the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...