Metal-free photosensitized radical relay 1,4-carboimination across two distinct olefins†
Abstract
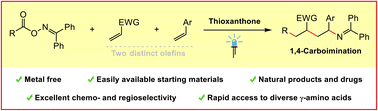
Intermolecular carboamination of olefins offers a powerful platform for the rapid construction of structurally complex amines from abundant feedstocks. However, these reactions often require transition-metal catalysis, and are mainly limited to 1,2-carboamination. Herein, we report a novel radical relay 1,4-carboimination across two distinct olefins with alkyl carboxylic acid-derived bifunctional oxime esters via energy transfer catalysis. The reaction is highly chemo- and regioselective, and multiple C–C and C–N bonds were formed in a single orchestrated operation. This mild and metal-free method features a remarkably broad substrate scope with excellent tolerance of sensitive functional groups, therefore providing easy access to structurally diverse 1,4-carboiminated products. Moreover, the obtained imines could be easily converted into valuable biologically relevant free γ-amino acids.


 Please wait while we load your content...
Please wait while we load your content...