Diazazethrene bisimide: a strongly electron-accepting π-system synthesized via the incorporation of both imide substituents and imine-type nitrogen atoms into zethrene†
Abstract
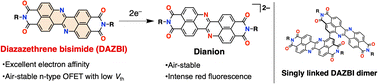
The development of highly electron-accepting π-systems is a fundamentally challenging issue despite their potential applications as high-performance n-type organic semiconductors, organic rechargeable batteries, and stable redox-active organocatalysts. Herein, we demonstrate that the incorporation of both imide substituents and imine-type nitrogen atoms into zethrene affords the strongly electron-accepting π-system diazazethrene bisimide (DAZBI). DAZBI has a low-lying LUMO (−4.3 eV vs. vacuum) and is readily reduced by the weak reductant L-ascorbic acid to afford the corresponding dihydro species. The injection of two electrons into DAZBI provides the corresponding dianion. These reduced species display remarkable stability, even under ambient conditions, and an intense red fluorescence. A DAZBI dimer, which was also synthesized, effectively accommodated four electrons upon electron injection.


 Please wait while we load your content...
Please wait while we load your content...