Regiodivergent metal-catalyzed B(4)- and C(1)-selenylation of o-carboranes†
Abstract
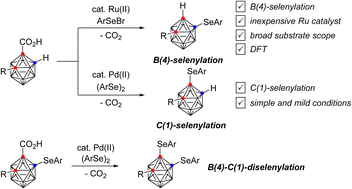
Regiodivergent transition metal-catalyzed B(4)- and C(1)-selenylation reactions of o-carboranes have been demonstrated. Namely, Ru(II)-catalysis selectively generated B(4)-selenylated o-carboranes from the reaction of o-carborane acids with arylselenyl bromides with the release of carbon dioxide. In contrast, Pd(II) catalysis provided exclusively C(1)-selenylated o-carboranes from the decarboxylative reaction of o-carborane acids with diaryl diselenides. In contrast to previous milestones in this area, these reactions demonstrate broad substrate scope with excellent yields. Combination of these methods leads to the formation of B(4)–C(1)-diselenylated o-carboranes. DFT studies revealed the mechanism of the Ru-process, with initial selenylation of the carborane cluster discovered to be essential for an energetically reasonable decarboxylation. This results in selenylation on the B(4) position prior to the decarboxylation event at C(1). This contrasted with the Pd-process in which the ready decarboxylation at C(1) leads to selenylation at C(1).


 Please wait while we load your content...
Please wait while we load your content...