A bioinspired, one-step total synthesis of peshawaraquinone†
Abstract
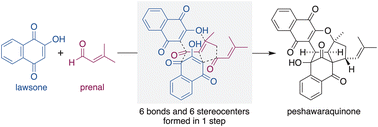
A concise synthesis of a stereochemically complex meroterpenoid, peshawaraquinone, via the unsymmetrical dimerization of its achiral precursor, dehydro-α-lapachone, is reported. Enabled by reversible oxa-6π-electrocyclizations of 2H-pyran intermediates, the base-catalyzed dimerization sets up an intramolecular (3 + 2) cycloaddition, with the formation of six stereocenters during the cascade. Combining the generation and in situ dimerization of dehydro-α-lapachone allows a one-step total synthesis of peshawaraquinone from lawsone and prenal.


 Please wait while we load your content...
Please wait while we load your content...